Tailor-made enzymes direct DNA repair pathways in the cell
RESEARCH HIGHLIGHTS
11 March - The team of Prof. Helle Ulrich at the Institute of Molecular Biology (IMB) in Mainz, Germany, has created a new strategy to manipulate polyubiquitin chains in cells using tailor-made enzymes. This method allows researchers to directly investigate the functional effects of specific changes in protein polyubiquitylation, and hence provide an important tool to help researchers understand the role of ubiquitylation in protein regulation. The results were published in the journal Molecular Cell.
Within the cell, proteins must be carefully regulated to ensure they are transported to the right locations, while proteins that are damaged or no longer needed must be removed. To do this, cells “earmark” their proteins with chemical tags called posttranslational modifications. Some posttranslational modifications mark proteins for transport to specific locations in the cell, while others mark unnecessary proteins or those that have completed their tasks for degradation and removal.
One such important posttranslational modification is ubiquitin. Proteins can be tagged with a single ubiquitin, or they can be polyubiquitylated by so-called ubiquitin ligase enzymes, which join additional ubiquitin groups to the first ubiquitin like links on a chain. Depending on the attachment site at which the ubiquitin groups are joined together, the resulting polyubiquitin chain can adopt various different shapes, ranging from extended and flexible to compact and folded.
Scientists have long noticed that polyubiquitin chains with different shapes are associated with distinct protein fates, suggesting that the cell may direct proteins toward different pathways by tagging them with particular varieties of chains. However, this was difficult to prove because there was no way to directly manipulate polyubiquitin chains in the cell, making it nearly impossible to know if a change in protein stability or location was actually caused by a change in the structure of the polyubiquitin chain.
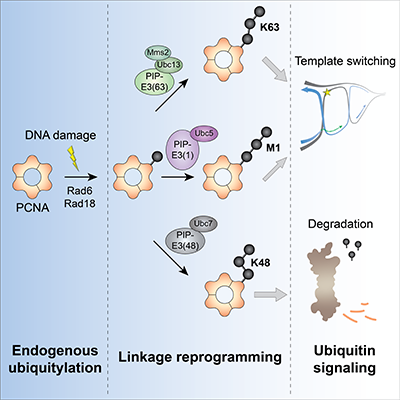
To tackle this problem, researchers in the lab of Prof. Ulrich, with key support from the group of Prof. Petra Beli, created and validated three tailor-made ubiquitin ligase enzymes. All three enzymes specifically extend ubiquitin chains on the same site of a protein called PCNA, which controls DNA replication and repair pathways, but each enzyme creates a polyubiquitin chain with a different shape.
The researchers then tested whether the three ubiquitin ligase enzymes caused different effects on PCNA function in yeast. Tagging PCNA with the “original” chain type that is normally used by the cell promoted DNA repair via a pathway called template switching and protected cells from DNA damage, while tagging PCNA with an alternative chain type that is known to promote protein degradation in other contexts appeared to inhibit DNA repair by promoting degradation of PCNA. Consequently, the cells became more sensitive to DNA damage. Surprisingly, tagging PCNA with a third chain type that is not naturally formed in yeast also promoted DNA repair by template switching, albeit not as effectively as the original chain type. The researchers reasoned that this may be because these two chains share a similar structure.
These results are important because they proved for the first time that tagging a protein with polyubiquitylation chains of different shapes at the same site can have different effects on its function. To show that this new method also works on other proteins, the researchers then used the same approach to design ubiquitin ligase enzymes targeting an unrelated protein, GFP. Prof. Ulrich says, “Our strategy has the potential to greatly expand the ways in which polyubiquitylation can be studied and should be applicable to a wide range of proteins and polyubiquitin chains”. Through this new technique, Prof. Ulrich and Prof. Beli have created a new tool for researchers to gain insights into how ubiquitylation controls proteins in the cell.
Further details
Cheryl Li is a Science Writer at the Institute of Molecular Biology (IMB).
Further information can be found at https://www.sciencedirect.com/science/article/pii/S1097276522001393
Helle Ulrich is a Scientific Director at the Institute of Molecular Biology (IMB) and a Professor of Biology at Johannes Gutenberg University Mainz. Further information about research in the Ulrich lab can be found atwww.imb.de/ulrich
Petra Beli is an Adjunct Director at the Institute of Molecular Biology (IMB) and a Professor of Biology at Johannes Gutenberg University Mainz. Further information about research in the Beli lab can be found at www.imb.de/beli
About the Institute of Molecular Biology gGmbH
The Institute of Molecular Biology gGmbH (IMB) is a centre of excellence in the life sciences that was established in 2011 on the campus of Johannes Gutenberg University Mainz (JGU). Research at IMB focuses on the cutting-edge fields of epigenetics, genome stability, ageing and RNA biology. The institute is a prime example of successful collaboration between a private foundation and government: The Boehringer Ingelheim Foundation has committed 154 million euros to be disbursed from 2009 until 2027 to cover the operating costs of research at IMB. The State of Rhineland-Palatinate has provided approximately 50 million euros for the construction of a state-of-the-art building and is giving a further 52 million in core funding from 2020 until 2027. For more information about IMB, please visit: www.imb.de.
About Johannes Gutenberg University Mainz
Johannes Gutenberg University Mainz (JGU) is a globally recognized research-driven university with around 31,000 students from over 120 nations. Its core research areas are in particle and hadron physics, the materials sciences, and translational medicine. JGU's success in Germany's Excellence Strategy program has confirmed its academic excellence: In 2018, the research network PRISMA+ (Precision Physics, Fundamental Interactions and Structure of Matter) was recognized as a Cluster of Excellence – building on its forerunner, PRISMA. Moreover, excellent placings in national and international rankings as well as numerous honors and awards demonstrate the research and teaching quality of Mainz-based researchers and academics. Further information at www.uni-mainz.de/eng
Boehringer Ingelheim Foundation
The Boehringer Ingelheim Foundation is an independent, non-profit organization that is committed to the promotion of the medical, biological, chemical, and pharmaceutical sciences. It was established in 1977 by Hubertus Liebrecht (1931–1991), a member of the shareholder family of the Boehringer Ingelheim company. Through its Perspectives Programme Plus 3 and its Exploration Grants, the Foundation supports independent junior group leaders. It also endows the international Heinrich Wieland Prize, as well as awards for up-and-coming scientists in Germany. In addition, the Foundation funds institutional projects in Germany, such as the Institute of Molecular Biology (IMB), the department of life sciences at the University of Mainz, and the European Molecular Biology Laboratory (EMBL) in Heidelberg.
Press contact for further information
Dr Ralf Dahm, Director of Scientific Management
Institute of Molecular Biology gGmbH (IMB), Ackermannweg 4, 55128 Mainz, Germany
Phone: +49 (0) 6131 39 21455, Email: press(at)imb.de